Updated on January 22nd, 2021
As we have previously written, education and information are key factors in the management of infectious disease. COVID-19 has resulted in an ’infodemic’ which has been subject to both misinformation and disinformation. It is likely that the roll out of vaccines will see an increase in disinformation. We will continue to update this page as we accumulate data.
Test, trace, isolate and the use of intermittent enhanced social distancing measures has been shown to be highly effective in controlling the impact of COVID-19 on the Hong Kong Health system. It has however resulted in a significant economic cost. We have previously described the management of any infectious epidemic as a balance of the lives lost due to the disease itself (and especially in COVID-19 due to the overwhelming of health systems) versus the harm done and the lives lost due to the economic impact of the public health measures. Immunisation offers the best chance to escape the current suppression but there is again a balance to be achieved in terms of the choice of new vaccines and the speed and prioritisation of the population immunisation program. The Hong Kong Government has announced the procurement of three different vaccine candidates which will be dependent upon peer-reviewed evidence from ongoing studies. This makes perfect sense. Voluntary immunization programs rely upon trust in Health institutions in addition to logistical factors including manufacture, procurement, delivery, cold storage and ultimately vaccination.
The distribution of all COVID-19 vaccines will be managed via the government in the first instance. We are not yet sure what our role will be in the delivery of COVID-19 vaccinations, but in anticipation of managing what will invariably be a logistical challenge, we have undertaken significant IT development. If you would like to be included in our COVID-19 vaccination database, please complete this brief form. We provide the option to review your current vaccination status and will provide further updates by email as we receive information about the vaccine rollout. Register for interest here. The impact of the vaccination program on the population will be maximised if vaccinations can be given in an equitable manner based upon clinical need. This will almost certainly be dependent on age, occupational and clinical risk. The health department has announced that the program will be voluntary and individuals will be able to choose which vaccine they wish to take.
OT&P has internationally accredited ACHS systems, a dedicated pharmacist and the ability to store vaccines at ultra-low temperatures. We will provide further information regarding our role in the vaccination program as the situation evolves.
‘Vaccines don’t save lives vaccinations save lives’
The following vaccinations have been sourced by the Hong Kong government:
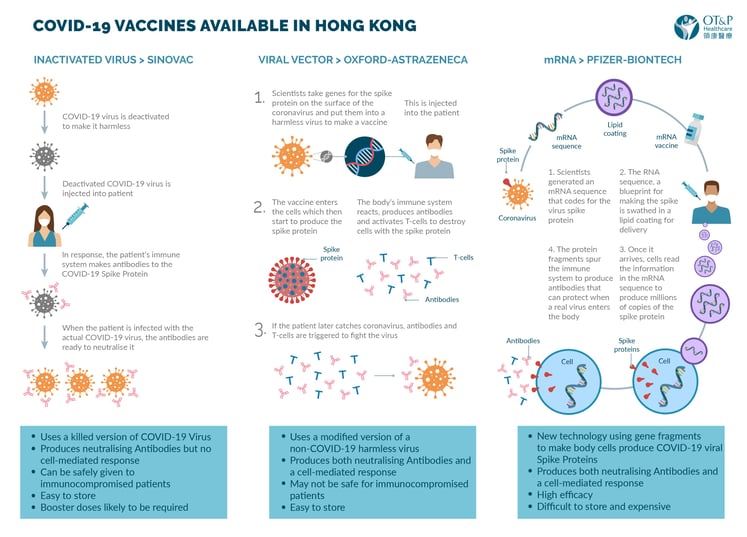
- The Pfizer-BioNTech vaccine: This is an mRNA vaccine. It represents a new technology in which a small segment of mRNA is injected into the body. This causes the body to produce spike protein and leads to the development of immunity. This vaccine (along with the Moderna vaccine which is also mRNA) report the highest efficacy rates[1]. It has been given emergency licensing approval by a number of countries. This is a new technology and safety and efficacy data has recently been published in the New England Journal of Medicine[2]. The vaccine must be stored at very low temperatures so the maintenance of the cold chain will be a logistical challenge. Vaccination involves 2 shots 21 days apart. In Hong Kong the data sheet for the vaccine given can be found here.
- Sinovac Biotech: This is a more traditional inactivated vaccine. The phase 1 and 2 trials were reported in the Lancet[3]. The phase 3 trials are due to report shortly. This vaccine will be able to be stored in a standard fridge making widespread use easier than the mRNA vaccines. It is given as 2 shots 14 days apart. Initial press reported suggested that the vaccine had been used extensively with good evidence of efficacy. Recent press reports suggested disappointing efficacy of around 50% from a study in Brazil.
- Oxford-AstraZeneca: This is a viral vector vaccine using an engineered adenovirus. The phase 3 studies have been reported in the Lancet showing efficacy between 62 - 90% depending upon the dose[4]. The lower initial dose produced a better response. This counterintuitive finding may be explained by differences in the population (generally younger) or may represent a true finding. This will require further study. The vaccine is given as 2 shots 28 days part although the trials are also looking at single shots and the impact of different doses. AstraZeneca vaccine can be stored in a standard fridge and the company is running the project as a not-for-profit. This vaccine is likely to be used widely in developing nations.
[Click here for full-sized image]
Developing a COVID-19 Vaccine
The development of the COVID-19 vaccine candidates has occurred at an unprecedented rate. There is still much that we do not know[5]. Normally vaccines develop through three phases of trials. These trials are mostly designed to answer a couple of questions.
1. Is the vaccine safe?
2. Is the vaccine effective in preventing disease and/or reducing disease severity?
The vaccine trials for COVID-19 have been powered to identify a reduction in disease. These studies are very expensive to undertake and typically recruit around 30,000 volunteers. They are designed to complete if they identify around 150 cases. The cases are confirmed by PCR testing but they are generally symptomatic, mostly mildly symptomatic although there have been some more serious disease. Regardless, the studies show the vaccine candidates to be generally safe within the numbers involved and to show effectiveness in reducing cases of illness. It will be important to analyse the final reported phase 3 trails closely. Studies with different end points (clinical severe, moderate, mild and asymptomatic disease) will produce different values. The studies all have slightly different methods and headline numbers such as 95% Pfizer versus 50% Sinovac are not necessarily comparing like with like. This article describes the dilemmas and common pitfalls of interpreting vaccine trials[6].
There are other questions which the phase 3 trials are not necessarily able to answer yet. The questions which require further ongoing research include:
3. Is the vaccine effective in reducing or preventing transmission of the virus?
4. How long is the vaccine effective and will booster doses be required?
5. How does the vaccine behave in different populations?
Reducing the likelihood of an individual developing severe illness is obviously very important. Only with greater data will we understand whether the vaccines also reduce transmission and asymptomatic infections. This would be important in increasing the effectiveness of vaccination in controlling the epidemic. Much more data is necessary in order to identify rarer complications. We also need longer term studies in order to fully understand the length of immunity and the need for boosters. Finally, we need to better understand the effectiveness of the vaccines in different populations including differences of age and ethnicity.
The development of vaccine candidates represents an incredible scientific achievement. The new mRNA vaccines in particular represent an enormous leap in vaccine development in terms of management of current and future infectious disease. If they work as well as some of the early data suggests and challenges to distribution and storage can be overcome, then we may genuinely be seeing one of the great medical advances of our lifetime. However, we are still early in the process and more data is required both on safety and efficacy. A recent editorial in the Lancet Microbe is entitled COVID-19: The Pandemic will not end overnight[7]. There are many other vaccine candidates in different stages of research. The World Health Organisation keeps an updated list of vaccines under development[8]. As we accumulate more data it may be that other vaccines may offer advantages in terms of safety, effectiveness or in the ability to impact the spread of the disease in addition to reducing disease severity. Understanding the different factors will require continued research and surveillance.
We will continue to update this page as further evidence is published.
Reference
- Moderna's COVID-19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study. (n.d.). Retrieved December 14, 2020, from https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy
- Polack, F., Al., E., For the C4591001 Clinical Trial Group*, Author AffiliationsFrom Fundacion INFANT (F.P.P.) and iTrials-Hospital Militar Central (G.P.M.), Longo, E., F. P. Polack and Others, . . . E. J. Rubin and D. L. Longo. (2020, December 10). Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine: NEJM. Retrieved December 21, 2020, from https://www.nejm.org/doi/10.1056/NEJMoa2034577
- Safety, tolerability, and immunogenicity of an inactivated ... (n.d.). Retrieved December 14, 2020, from https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30843-4/fulltext
-
Safety and efficacy of the ChAdOx1 nCoV-19 vaccine ... (n.d.). Retrieved December 15, 2020, from https://www.thelancet.com/lancet/article/s0140-6736(20)32661-1
- Doshi, P. (2020, October 21). Will covid-19 vaccines save lives? Current trials aren't designed to tell us. Retrieved December 15, 2020, from https://www.bmj.com/content/371/bmj.m4037
- New Vaccine Data Is Coming: Watch Out for These 3 Claims (n.d.). Retrieved January 22, 2021, from https://www.wired.com/story/new-vaccine-data-is-coming-watch-out-for-these-3-claims/
- COVID-19 vaccines: The pandemic will not end overnight ... (n.d.). Retrieved December 21, 2020, from https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(20)30226-3/fulltext
- Draft landscape of COVID-19 candidate vaccines. (n.d.). Retrieved December 15, 2020, from https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
 Central General Practice
Central General Practice
 Repulse Bay
Repulse Bay
 Clearwater Bay
Clearwater Bay
 BodyWorX Clinic
BodyWorX Clinic
 Central Specialist Clinic
Central Specialist Clinic
 MindWorX Clinic
MindWorX Clinic
 Partner Clinics
Partner Clinics
 Family Clinic
Family Clinic
 OT&P Annerley Midwives Clinic
OT&P Annerley Midwives Clinic






